|
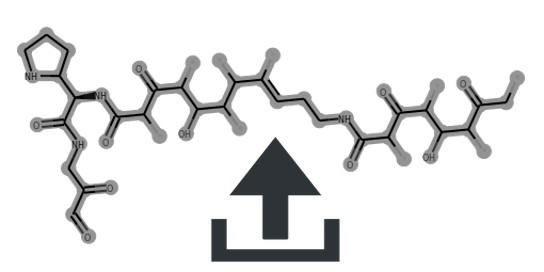
fusarin |
15 |
0.4 |
0.44 |
0.47 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C/C=C(C)\C=C(C)\C=C(C)\C=C\C=C(/C)C(=O)[C@]12O[C@H]1[C@@](O)(CCO)NC2=O |
view |
fusarin |
|
|
coronatine |
0 |
0.53 |
0.48 |
0.7 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC[C@H]1C[C@@]1(NC(=O)C1=C[C@H](CC)C[C@@H]2C(=O)CC[C@H]12)C(=O)O |
view |
coronatine |
|
|
Xenocoumacin II |
1 |
0.48 |
0.43 |
0.63 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)CC(NC(=O)C(O)C(O)C1CCCN1)C1Cc2cccc(O)c2C(=O)O1 |
view |
Xenocoumacin II |
|
|
Wortmanamide |
2 |
0.45 |
0.42 |
0.59 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C[C@@H](O)C/C=C/CCCCCCCC/C=C/C(=O)NCCCCC(=O)O |
view |
Wortmanamide |
|
|
9-methylstreptimidone |
3 |
0.45 |
0.49 |
0.54 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C/C=C\C(C)=C\[C@H](C)C(=O)C[C@H](O)CC1CC(=O)NC(=O)C1 |
view |
9-methylstreptimidone |
|
|
belactosin A |
4 |
0.45 |
0.48 |
0.54 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC[C@H](C)[C@@H]1C(=O)O[C@H]1C(=O)N[C@H]1C[C@H]1C[C@H](NC(=O)[C@H](C)N)C(=O)O |
view |
belactosin A |
|
|
tambromycin |
5 |
0.44 |
0.44 |
0.39 |
0.67 |
Cl:1/2 |
MIBiG |
Source |
Cn1cc(C2=N[C@](C)(C(=O)N[C@H](C(=O)N[C@@](C)(CO)C(=O)O)[C@H]3CCCN3)CO2)c2c(O)cc(Cl)cc21 |
view |
tambromycin |
|
|
geldanamycin |
6 |
0.43 |
0.42 |
0.55 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
COC1=C2C[C@@H](C)C[C@H](OC)[C@H](O)[C@@H](C)/C=C(/C)[C@H](OC(N)=O)[C@@H](OC)/C=C\C=C(\C)C(=O)NC(=CC1=O)C2=O |
view |
geldanamycin |
|
|
Rakicidin D |
7 |
0.43 |
0.42 |
0.55 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C=C1/C=C\C(=O)N(C)CC(=O)N[C@@H]([C@H](O)C(N)=O)C(=O)OC([C@H](C)CCCCCC)[C@H](C)C(=O)N1 |
view |
Rakicidin D |
|
|
valclavam |
8 |
0.42 |
0.48 |
0.48 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)C(N)C(=O)NC(C(=O)O)C(O)CC1CN2C(=O)CC2O1 |
view |
valclavam |
|
|
tabtoxin |
9 |
0.41 |
0.42 |
0.51 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C[C@@H](O)[C@H](NC(=O)[C@@H](N)CC[C@]1(O)CNC1=O)C(=O)O |
view |
tabtoxin |
|
|
myxalamid |
10 |
0.41 |
0.45 |
0.49 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(/C=C/C=C\C=C\C=C(/C)C(=O)N[C@@H](C)CO)=C\[C@@H](C)[C@@H](O)/C(C)=C/C(C)C |
view |
myxalamid |
|
|
belactosin C |
11 |
0.41 |
0.46 |
0.47 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC[C@H](C)[C@@H]1C(=O)O[C@H]1C(=O)NCCC[C@H](NC(=O)[C@H](C)N)C(=O)O |
view |
belactosin C |
|
|
botryenalol |
12 |
0.4 |
0.42 |
0.49 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(=O)O[C@H]1C[C@@H](C)C(C=O)=C2[C@@H]1C(C)(C)C[C@]2(C)CO |
view |
botryenalol |
|
|
phytocassane |
13 |
0.4 |
0.43 |
0.48 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C=CC1=CC(=O)[C@H]2[C@@H](CC[C@H]3C(C)(C)C(=O)[C@@H](O)C[C@]23C)[C@H]1C |
view |
phytocassane |
|
|
Pellasoren |
14 |
0.4 |
0.42 |
0.48 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC/C=C(\OC)C(=O)N[C@@H](C)/C=C(C)/C=C(C)/C=C/C[C@H](C)[C@H]1OC(=O)[C@H](C)C[C@@H]1C |
view |
Pellasoren |
|
|
lankacidin |
16 |
0.4 |
0.46 |
0.45 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(=O)C(=O)N[C@@H]1/C=C(C)\C=C/[C@@H](O)C/C=C(C)\C=C/[C@@H](O)C[C@H]2OC(=O)[C@]1(C)C(=O)[C@@H]2C |
view |
lankacidin |
|
|
actinonin |
17 |
0.4 |
0.46 |
0.45 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)N1CCC[C@H]1CO)C(C)C |
view |
actinonin |
|
|
ebelactone |
18 |
0.39 |
0.45 |
0.44 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC[C@@H](C)[C@@H](O)[C@H](C)C(=O)[C@H](C)/C=C(\C)C[C@H](C)[C@@H]1OC(=O)[C@H]1C |
view |
ebelactone |
|
|
Thailanstatin A |
19 |
0.38 |
0.44 |
0.44 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(=O)O[C@@H](C)/C=C\C(=O)N[C@@H]1C[C@H](C)[C@H](C/C=C(C)/C=C/[C@H]2O[C@H](CC(=O)O)C[C@@]3(CO3)[C@@H]2O)O[C@@H]1C |
view |
Thailanstatin A |
|
|
Malleilactone |
20 |
0.38 |
0.42 |
0.44 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CCCCCCC/C=C(C)/C(O)=C1\C=C(C(=O)CC)OC1=O |
view |
Malleilactone |
|
|
syringolin A |
21 |
0.38 |
0.42 |
0.44 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)C1/C=C\C(=O)NCC/C=C\C(NC(=O)C(NC(=O)NC(C(=O)O)C(C)C)C(C)C)C(=O)N1 |
view |
syringolin A |
|
|
13-epi-Dorrigocin A |
22 |
0.38 |
0.45 |
0.42 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CO[C@@H](/C=C/CC/C=C/C(=O)O)[C@@H](O)[C@H](C)/C=C(\C)[C@@H](O)[C@H](C)C(=O)CCCC1CC(=O)NC(=O)C1 |
view |
13-epi-Dorrigocin A |
|
|
Dorrigocin A |
23 |
0.38 |
0.45 |
0.42 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CO[C@@H](/C=C/CC/C=C/C(=O)O)[C@@H](O)[C@H](C)/C=C(\C)[C@H](O)[C@H](C)C(=O)CCCC1CC(=O)NC(=O)C1 |
view |
Dorrigocin A |
|
|
Lactimidomycin |
24 |
0.37 |
0.42 |
0.43 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C/C(=C\[C@H](C)C(=O)C[C@H](O)CC1CC(=O)NC(=O)C1)[C@@H]1OC(=O)/C=C\CC/C=C\C=C/[C@@H]1C |
view |
Lactimidomycin |
|
|
8,9-dihydro-LTM |
25 |
0.37 |
0.42 |
0.43 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C/C(=C\[C@H](C)C(=O)C[C@H](O)CC1CC(=O)NC(=O)C1)[C@@H]1OC(=O)/C=C\CC/C=C\CC[C@@H]1C |
view |
8,9-dihydro-LTM |
|
|
delta-(L-alpha-aminoadipyl)-L-cysteine-D-valine |
26 |
0.37 |
0.43 |
0.42 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)[C@@H](NC(=O)[C@H](CS)NC(=O)CCC[C@H]([NH3+])C(=O)[O-])C(=O)[O-] |
view |
delta-(L-alpha-aminoadipyl)-L-cysteine-D-valine |
|
|
coelimycin P1 |
27 |
0.37 |
0.45 |
0.41 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C/C=C/C(=O)/C1=C/C(=C2/C=CCCN2)SCC(NC(C)=O)C(=O)O1 |
view |
coelimycin P1 |
|
|
isopenicillin N |
28 |
0.37 |
0.44 |
0.41 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC1(C)S[C@@H]2[C@H](NC(=O)CCC[C@H](N)C(=O)O)C(=O)N2[C@H]1C(=O)O |
view |
isopenicillin N |
|
|
Dorrigocin B |
29 |
0.37 |
0.45 |
0.4 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CO[C@@H](/C=C/CC/C=C/C(=O)O)[C@@H](O)[C@H](C)[C@@H](O)/C(C)=C/[C@H](C)C(=O)CCCC1CC(=O)NC(=O)C1 |
view |
Dorrigocin B |
|
|
eponemycin |
30 |
0.37 |
0.49 |
0.37 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C=C(C)C[C@H](NC(=O)C(CO)NC(=O)CCCCC(C)C)C(=O)[C@@]1(CO)CO1 |
view |
eponemycin |
|
|
Kalimantacin A |
31 |
0.36 |
0.43 |
0.39 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C=C(CCC=CC=CCC(C)CC(=O)CC(O)CNC(=O)C(C)C(C)OC(N)=O)CC(C)CC(C)=CC(=O)O |
view |
Kalimantacin A |
|
|
galbonolide A |
32 |
0.36 |
0.44 |
0.38 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C=C1/C=C(/C)[C@H](CC)OC(=O)[C@H](C)C(=O)C(O)(CO)C/C(OC)=C\[C@@H](C)C1 |
view |
galbonolide A |
|
|
tirandamycin |
33 |
0.36 |
0.44 |
0.38 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(/C=C/C(=O)C1=C(O)NCC1=O)=C\[C@@H](C)[C@H]1O[C@]2(C)O[C@@H](C(=O)[C@@H]3O[C@@]32C)[C@@H]1C |
view |
tirandamycin |
|
|
cephalosporin C |
34 |
0.36 |
0.44 |
0.38 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(=O)OCC1=C(C(=O)O)N2C(=O)[C@@H](NC(=O)CCC[C@@H](N)C(=O)O)[C@H]2SC1 |
view |
cephalosporin C |
|
|
Abyssomicin C |
35 |
0.35 |
0.42 |
0.39 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C[C@@H]1C/C=C\[C@@H]2[C@@H](O)[C@@H]3OC4=C(C(=O)O[C@]42C[C@H]3C)C(=O)[C@H](C)C1 |
view |
Abyssomicin C |
|
|
4´-oxomacrophorin A |
36 |
0.35 |
0.42 |
0.39 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C=C1CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1C[C@@]12O[C@@H]1C(=O)C(CO)=CC2=O |
view |
4´-oxomacrophorin A |
|
|
monascin |
37 |
0.35 |
0.42 |
0.39 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C/C=C/C1=CC2=C(CO1)C(=O)[C@]1(C)OC(=O)[C@H](C(=O)CCCCC)[C@H]1C2 |
view |
monascin |
|
|
jerangolid A |
38 |
0.35 |
0.42 |
0.39 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC[C@H]1O[C@@H](/C(C)=C/[C@H](C)/C=C/[C@H]2CC(OC)=C(CO)C(=O)O2)CC=C1C |
view |
jerangolid A |
|
|
botcinic acid |
39 |
0.35 |
0.43 |
0.37 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CCCC[C@H](O)/C=C/C(=O)O[C@H]1[C@H](C)O[C@](C)([C@@H](O)[C@@H](C)C(=O)O)[C@@H](O)[C@@H]1C |
view |
botcinic acid |
|
|
cystargolide A |
40 |
0.35 |
0.46 |
0.35 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H]1OC(=O)[C@H]1C(C)C)C(C)C)C(=O)O |
view |
cystargolide A |
|
|
cystargolide B |
41 |
0.34 |
0.45 |
0.35 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H]1OC(=O)[C@H]1C(C)C)C(C)C)C(=O)O |
view |
cystargolide B |
|
|
4´-oxomacrophorin E |
42 |
0.34 |
0.44 |
0.35 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C=C1CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1C[C@@]12O[C@@H]1C(=O)C(COC(=O)CC(=O)O)=CC2=O |
view |
4´-oxomacrophorin E |
|
|
Spiruchostatin A |
43 |
0.33 |
0.42 |
0.35 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)[C@H]1NC(=O)[C@H]2CSSCC/C=C\[C@H](CC(=O)N[C@H](C)C(=O)N2)OC(=O)C[C@@H]1O |
view |
Spiruchostatin A |
|
|
yersiniabactin |
44 |
0.33 |
0.43 |
0.33 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)(C1=N[C@@](C)(C(=O)O)CS1)[C@H](O)[C@@H]1CSC([C@H]2CS/C(=C3\C=CC=CC3=O)N2)N1 |
view |
yersiniabactin |
|
|
herboxidiene |
45 |
0.32 |
0.42 |
0.33 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CO[C@@H]([C@@H](C)O)[C@@H](C)[C@H]1O[C@]1(C)C[C@H](C)/C=C/C=C(\C)[C@H]1O[C@@H](CC(=O)O)CC[C@@H]1C |
view |
herboxidiene |
|
|
coelichelin |
46 |
0.32 |
0.44 |
0.32 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
C[C@@H](O)[C@@H](NC(=O)[C@H](N)CCCN(O)C=O)C(=O)N(O)CCC[C@H](NC(=O)[C@H](N)CCCN(O)C=O)C(=O)O |
view |
coelichelin |
|
|
yanuthone D |
47 |
0.32 |
0.43 |
0.32 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CC(C)=CCC/C(C)=C/CC/C(C)=C/C[C@@]12O[C@@H]1C(=O)C(COC(=O)CC(C)(O)CC(=O)O)=CC2=O |
view |
yanuthone D |
|
|
fortimicin |
48 |
0.32 |
0.43 |
0.31 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CO[C@H]1[C@@H](O)[C@H](N)[C@@H](O[C@H]2O[C@H](C(C)N)CC[C@H]2N)[C@H](O)[C@@H]1N(C)C(=O)CN.O=S(=O)(O)O.O=S(=O)(O)O |
view |
fortimicin |
|
|
AF-toxin |
49 |
0.31 |
0.41 |
0.32 |
0.0 |
Cl:0/2 |
MIBiG |
Source |
CCC(C)C(OC(=O)C(O)C(C)(C)O)C(=O)OC(/C=C/C=C/C=C/C(=O)O)C1(C)CO1 |
view |
AF-toxin |
|